Which Best Describes the Nucleus of an Atom
AIEEE Bank Exams CAT. 483 x 1014 Hz.

Doe Explains Nuclei Department Of Energy
According to the standing wave model of the atom when the atom emits a photon its energy.

. Almost all the mass of an atom is located in the nucleus with a very small contribution from the electron shells. All the protons and neutrons are located in the nucleus. D a core of electrons and neutrons surrounded by protons.
What was on the photographic plate in Becquerels drawer with the uranium. What best describes the mass of an electron. An atom is a particle of matter that uniquely defines achemical element.
The nucleus is just a tiny part of the atom but it contains virtually all of the atoms mass. Which best describes the nucleus of a carbon C atom. What best describes the nucleus of an atom-.
The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons. Which answer best describes the nucleus. What best describes the nucleus of an atom-.
So there is 13 electrons surrounded the nucleus. The atomic number of Al atom is 13 which means that there is 13 protons in the nucleus. All atoms of the unstable nuclides listed in this table have.
The protons are positively charged which gives the nucleus a positive charge while the neutrons have no charge. Answer choices an image of the mineral. Answer Expert Verified The nucleus of an atom is a small dense region at the center of an atom that contains protons and neutrons.
B The nucleus occupies very little of the atoms volume and contains little of its mass. Decreases and the electron moves closer to the nucleus. A hydrogen atom is in an excited state.
The protons are positively charged which gives the nucleus a positive charge while the neutrons have no. Not significant to the overall mass of the atom. 79 atomic mass units.
Which best describes the nucleus of an atom. How many protons are in this atom if it has a balanced charge. It consists of positive protons and neutral neutrons so it has an overall positive charge.
A protons and electrons grouped together in a random pattern. Question 10 30 seconds Q. Eight protons six neutrons.
D All of the above. 145 x 1015 Hz. The planets describe the size of the nucleus of the atom compared to the whole atom.
The nucleus of an atom is the densest part of an atom. Hence the statement best describes the nucleus of an atom is It is centrally located structure comprising almost all of the atomic mass and having an overall positive charge It has the a charge of 13 and is surrounded by a total of 14 electrons. The nucleus of an atom is a small dense region at the center of an atom that contains protons and neutrons.
C It has protons. Six protons eight neutrons. Nuclide Stability C-12 Stable C-14 Unstable N-14 Stable N-16 Unstable.
Best describes the nucleus of an aluminum atom. The nucleus justifies for the atomic mass of an torm since the atomic mass is obtained by adding the number of protons and neutrons in the nucleus if an atom. Report an issue.
1Which of the following best describes an atom. It contains the protons and neutrons of an atom. The current model of an atom is best described by the Solar System.
All atomic nuclei are positively charged. Unit 9 - Physical Science - Acids and Bases. Which describes a nucleus.
Which statement best describes the nucleus of an atom. Protons and neutrons make up the nucleus of an atom. 10 protons four neutrons.
C a core of protons and neutrons surrounded by electrons. The total mass of the protons in an atom of gold-198 is approximately. A The nucleus occupies most of the atoms volume but contains little of its mass.
The current model of an atom is best described by the SolarSystem. Electrons travel in definite circular pathways around the nucleus. Each atom has a tiny nucleus at its center.
Analyst Bank Clerk Bank PO. The nucleus is a small dense region at the center of the atom. Each electron is negatively charged.
D All of the above. And the Al atom has net charge of 0. Answer choices It is a centrally located structure comprising almost all of the atomic mass and having an overall neutral charge It is a centrally located structure comprising almost none of.
Which of the following best describes the nucleus of an atom of carbon-14. The planets describe the size of the nucleus of the atomcompared to the whole atom. Nucleus of the Atom.
B protons and electrons grouped together in an alternating pattern. A It is the densest part of the atom B It has neutrons C It has protons D All of the above. Which best describes the nucleus of an atom.
Which statement best describes the density of an atoms nucleus. So the nucleus has a charge of 13. An atom consists of a central nucleus that is usually surrounded by one or more electrons.
C The nucleus occupies most of the atoms volume and contains most of its mass. Four protons 10 neutrons.
What Is An Atom And Atomic Nucleus Quora

Atom Rutherford S Nuclear Model Britannica
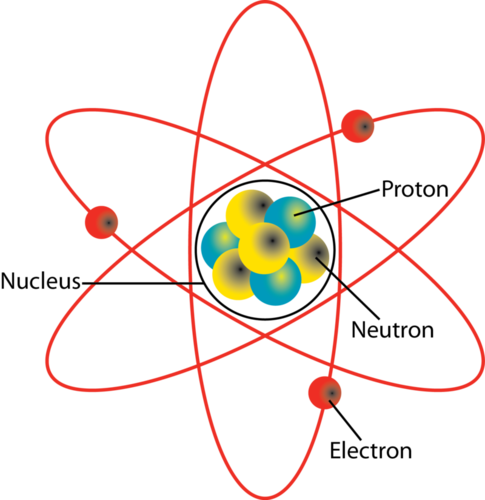
What Two Particles Are Found In The Nucleus Of An Atom Socratic

Comments
Post a Comment